When a pharmacist hands you a generic pill instead of the brand-name version, you might wonder: is this really the same? The answer isn’t just trust-it’s data. And that data lives in the FDA Orange Book. This isn’t some obscure government document. It’s the official, daily-updated database that tells pharmacists, doctors, and insurers exactly which generic drugs can be swapped for brand-name ones without risking your health. If you’re verifying a substitution, checking a formulary, or just trying to understand why your prescription changed, the Orange Book is where you need to look.
What the FDA Orange Book Actually Is
The FDA Orange Book, officially called Approved Drug Products with Therapeutic Equivalence Evaluations, has been around since 1980. But it became essential after the 1984 Hatch-Waxman Act, which created the modern system for approving generic drugs. The goal? Let safe, cheaper generics enter the market without forcing patients to pay more or risk inconsistent results. It lists over 16,000 FDA-approved drug products-both prescription and over-the-counter. But here’s the catch: only prescription drugs get therapeutic equivalence ratings. OTC meds? They’re listed, but no ratings. That’s because the FDA doesn’t evaluate OTC products for substitution like they do for prescriptions. The Orange Book doesn’t just say “this generic works.” It tells you why it works. It links each generic to its original brand-name drug-the Reference Listed Drug (RLD)-and assigns a code that says whether it’s safe to swap. That code is everything.Understanding Therapeutic Equivalence (TE) Codes
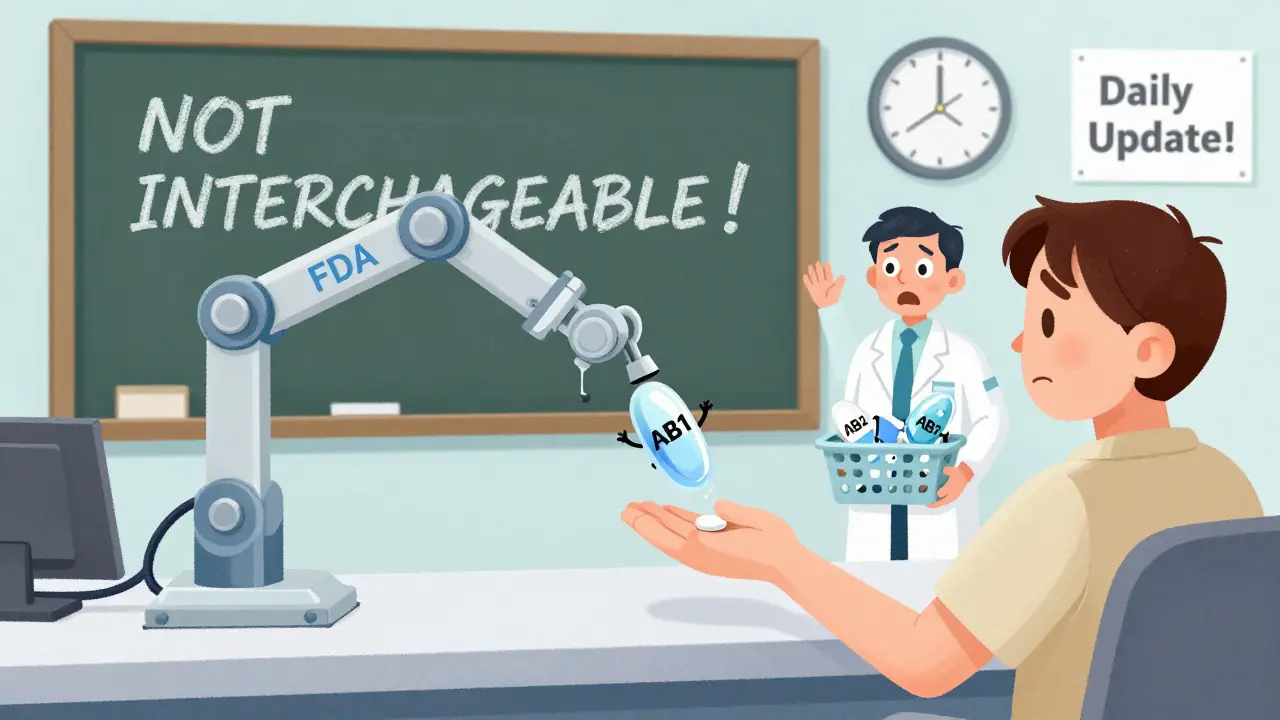
The TE code is a two-letter rating that tells you if a generic is interchangeable with the brand. It’s not marketing jargon-it’s a scientific determination. - AB: This is the gold standard. It means the generic has the same active ingredient, dosage form, strength, and route of administration as the brand. It’s also been proven bioequivalent-meaning your body absorbs it at the same rate and to the same extent. If a generic has an AB rating, it’s considered therapeutically equivalent and can be substituted in most states. - AB1, AB2, AB3: These mean the generic matches one of several possible RLDs. For example, if two different brand drugs exist for the same active ingredient (say, two versions of metoprolol), the generics are coded AB1 or AB2 to show which one they match. You can’t swap an AB1 generic for an AB2 product-they’re not interchangeable. - B: This is a red flag. A B rating means the FDA doesn’t consider the generic equivalent. This could be due to bioequivalence issues, formulation problems, or lack of testing. Never substitute a B-rated drug. - BX: This is a newer category for drugs with potential bioequivalence concerns, often narrow therapeutic index (NTI) drugs like warfarin or levothyroxine. Even if they’re AB-rated, some states require extra approval before substituting these. The FDA doesn’t assign these codes randomly. They require five strict conditions: approved safety and effectiveness, identical active ingredients, same dosage form and route, proven bioequivalence, proper labeling, and compliance with manufacturing standards. If any one fails, the drug gets a B or BX rating.How to Search the Electronic Orange Book
The paper version hasn’t been printed since 2007. Today, it’s all online: the Electronic Orange Book (EOB). It’s free, updated daily, and designed for real-world use. Here’s how to find what you need in five steps:- Start with the brand name. Type the brand name (like Synthroid or Lipitor) into the search bar. Don’t use the generic name yet-start with what you know.
- Find the active ingredient. The results will show you the active ingredient(s) in the brand drug. For Synthroid, that’s levothyroxine sodium.
- Do an ingredient search. Go back to the search page and select “Active Ingredient.” Enter the ingredient you just found. This pulls up every approved product with that ingredient.
- Filter by dosage form and route. You’ll see a long list. Sort by dosage form (tablet, capsule, injection) and route (oral, topical, inhalation). This is critical-equivalence only applies within the same form and route.
- Check the RLD and TE Code columns. The RLD column says “Yes” for the original brand. Everything else is a generic. The TE Code column shows the rating. Look for AB, AB1, AB2, etc. If you see “No” in the RLD column and “AB” in the TE Code, you’ve found a substitute.
What the Orange Book Doesn’t Tell You
The Orange Book is authoritative, but it’s not a complete picture. It doesn’t tell you about state laws, insurance rules, or patient-specific risks. For example: Levothyroxine (Synthroid) is AB-rated. But in many states, pharmacists can’t swap it without a doctor’s note-even if the generic is AB-rated. Why? Because it’s a narrow therapeutic index drug. Tiny changes in dose can cause serious problems. The Orange Book flags these with BX codes, but it doesn’t say “don’t substitute.” That’s up to state pharmacy boards. Also, patent and exclusivity dates are listed, but they’re not the same thing. A patent might expire, but the brand might still have market exclusivity (like for new chemical entities). Generic companies can’t enter until both are gone. Mixing these up can lead to false assumptions about availability. And discontinued drugs? They’re listed separately. If you can’t find a drug in the main list, check the Discontinued Drug Product List. It’s easy to miss.Common Mistakes and How to Avoid Them
Even experienced pharmacists make errors. Here are the top three:- Confusing TE codes with patent status. Just because a patent expired doesn’t mean a generic is approved. Check the TE Code. No code? It’s not approved for substitution.
- Assuming all AB-rated drugs are interchangeable. AB1 and AB2 are not the same. If your patient is on an AB1 generic, switching to an AB2 could cause issues.
- Trusting third-party sites without checking the source. Drugs.com, Micromedex, and others pull from the Orange Book-but they’re often 24 to 72 hours behind. For critical decisions, always go to the FDA’s official site.
When to Use the Orange Book
You should check the Orange Book when:- A patient asks why their prescription changed to a generic.
- A pharmacy suggests a substitution and you want to confirm it’s allowed.
- Your insurance switches your drug and you need to verify equivalence.
- You’re reviewing formulary options for a clinic or hospital.
- You’re researching why a generic hasn’t come to market yet.
What’s Next for the Orange Book
The FDA is modernizing the system. By 2024, they plan to integrate it with the Purple Book (for biologics) and move to a machine-readable format called SPL. That means apps, EHRs, and pharmacy systems will soon pull data directly from the FDA-no manual searches needed. But for now, the manual search remains the gold standard. And it’s still the only way to be 100% sure.Can I trust a generic drug if it has an AB rating?
Yes. An AB rating means the FDA has confirmed the generic is pharmaceutically and bioequivalent to the brand-name drug. It must contain the same active ingredient, dosage form, strength, and route of administration, and it must be absorbed by the body at the same rate and extent. Millions of patients safely use AB-rated generics every day. But always tell your doctor if you notice any changes in how you feel after switching.
Why are some generic drugs not AB-rated?
A drug gets a B or BX rating if the FDA finds issues with bioequivalence, formulation, or delivery. This can happen with complex drugs like inhalers, topical creams, or injectables, where small differences in inactive ingredients or manufacturing affect how the drug works. Some older generics were approved before modern testing standards and haven’t been re-evaluated. Always check the TE code before substituting.
Can I substitute a generic if my state requires a doctor’s note?
The Orange Book tells you if substitution is scientifically valid. But state laws control whether pharmacists can actually swap the drug. For example, many states require physician approval for substituting levothyroxine or warfarin-even if they’re AB-rated. Always check your state’s pharmacy board rules. The Orange Book doesn’t override state law.
Is the Electronic Orange Book free to use?
Yes. The FDA provides the Electronic Orange Book for free at https://www.accessdata.fda.gov/scripts/cder/ob/. No login, no subscription, no fees. Third-party sites may charge for access or bundle it into paid tools, but the official source is always free.
How often is the Orange Book updated?
The Electronic Orange Book is updated daily with new approvals, patent changes, and discontinued products. Major revisions happen monthly. But for critical decisions, always check the date of the last update on the site. Don’t rely on printed materials or third-party sources-they can be outdated by days or weeks.
What if I can’t find a drug in the Orange Book?
First, check the Discontinued Drug Product List. If it’s not there, the drug may not be FDA-approved, or it might be an OTC product (which aren’t rated). If you’re sure it’s a prescription drug, contact the FDA at [email protected]. They respond to 95% of inquiries within 48 business hours.

