When your pharmacist hands you a pill bottle with a different name than what your doctor wrote on the prescription, it’s not a mistake. It’s a generic version of your medication. And if you’ve ever looked at the label and seen three or four different generic brands for the same drug, you’re not alone. Deciding which one to take isn’t just about price-it’s about safety, consistency, and how your body responds. The good news? Most generics work just as well as the brand-name version. The tricky part? Not all generics are created equal.
What Makes a Generic Drug Really the Same?
Every generic drug must contain the exact same active ingredient, dose, and form as the brand-name drug. That means if you’re taking 10 mg of lisinopril, every generic version has 10 mg of lisinopril. No more, no less. The FDA requires this. But what happens after you swallow it? That’s where bioequivalence comes in.
Bioequivalence means your body absorbs the drug at nearly the same rate and to the same extent as the brand. The FDA says a generic must deliver the drug into your bloodstream within 80% to 125% of the brand’s levels. That sounds like a wide range-but in practice, most generics are within 5% of the original. A 2017 FDA analysis found that the average difference between brand and generic versions was just 3.5%. That’s tiny.
But here’s the catch: this doesn’t mean every generic is identical to every other generic. Two different generics for the same drug can vary slightly in how fast they dissolve, what fillers they use, or how they’re manufactured. These differences are usually harmless. But for some drugs, even small changes can matter.
When Do These Small Differences Actually Matter?
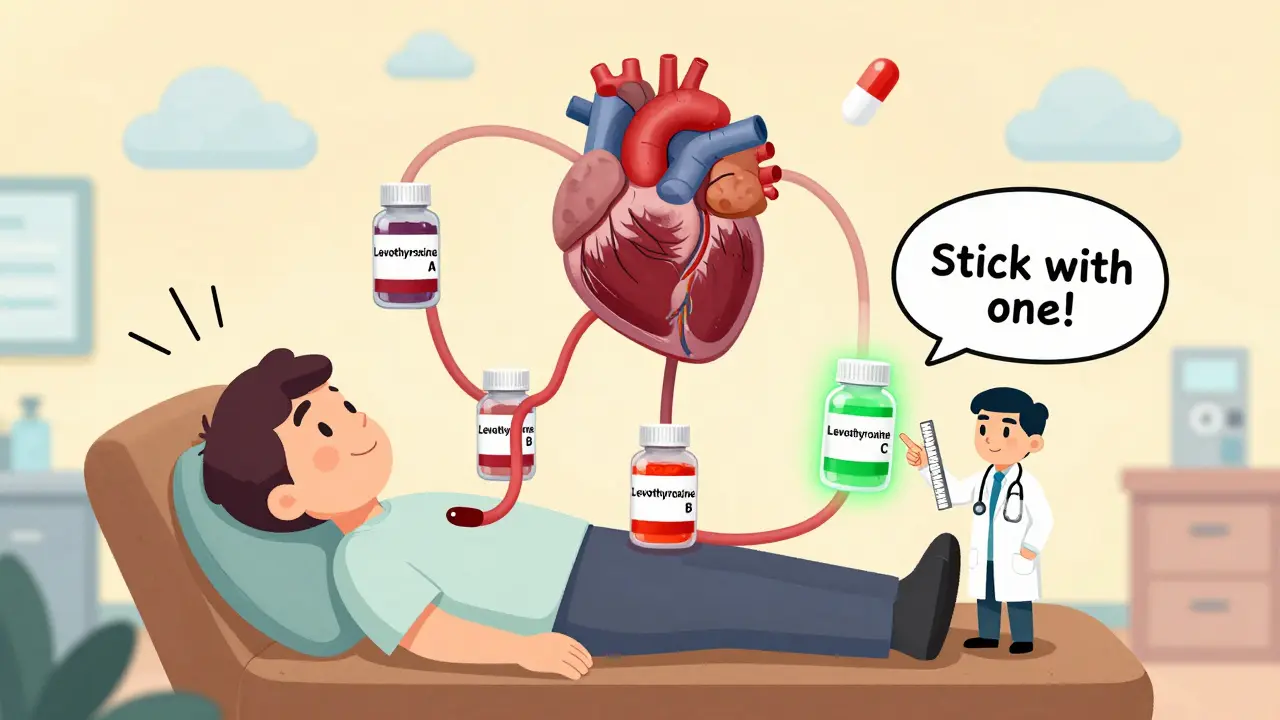
For most people, switching between generics is completely safe. But for certain medications-called drugs with a narrow therapeutic index (NTI)-even small changes in blood levels can cause problems. These are drugs where the difference between a helpful dose and a dangerous one is very small.
Examples include:
- Warfarin (blood thinner)
- Levothyroxine (thyroid hormone)
- Digoxin (heart medication)
- Phenytoin (seizure control)
For these, your doctor or pharmacist may recommend sticking with one manufacturer’s version once you’ve found what works. Why? Because switching between different generics-even if they’re all FDA-approved-can cause your blood levels to drift. That might mean your INR (for warfarin) goes too high, or your thyroid levels become unstable. One 2023 review from UCSF noted that patients on levothyroxine who switched between generics had higher rates of abnormal thyroid tests, even when all products were labeled as bioequivalent.
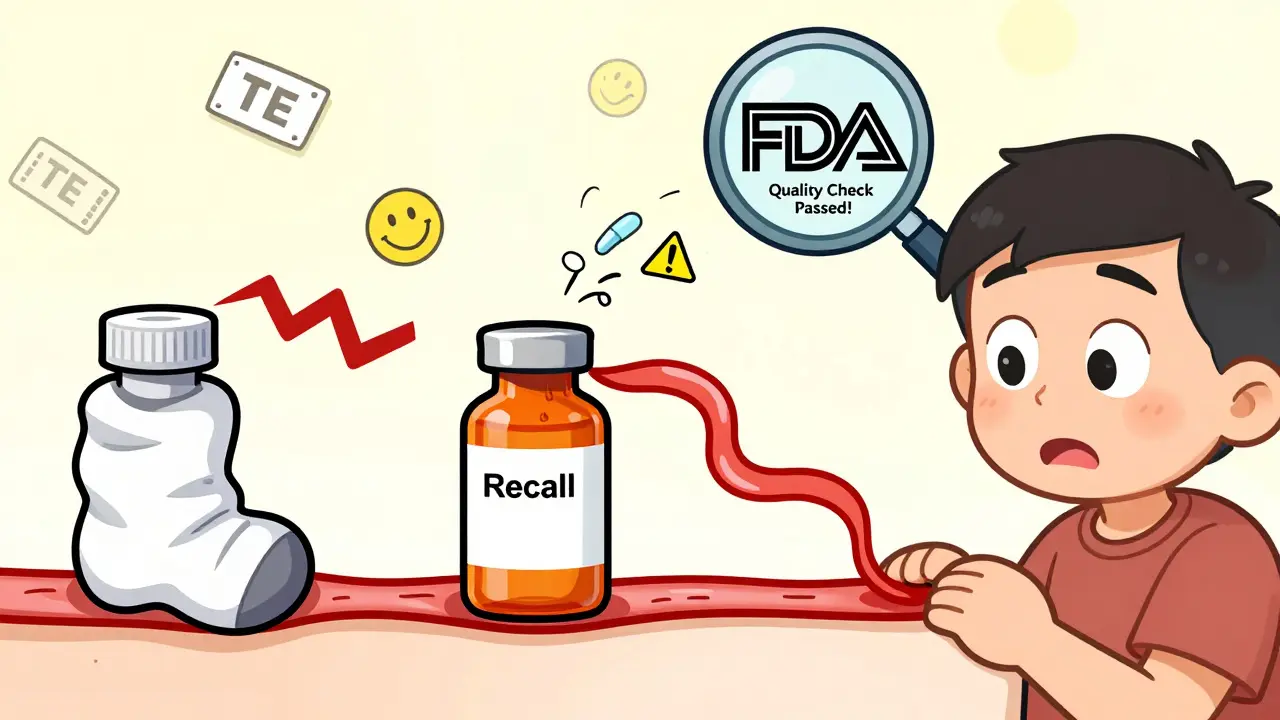
The FDA’s Orange Book lists each generic with a therapeutic equivalence (TE) code. Look for “AB” ratings. That means the generic is considered interchangeable with the brand. “B” ratings mean there’s uncertainty-maybe the bioequivalence data is limited, or the drug has a narrow therapeutic index. If you’re on an NTI drug, avoid B-rated generics unless your doctor specifically approves it.
How to Read the Orange Book (Without Being a Pharmacist)
You don’t need to be a doctor to check this. The FDA’s Orange Book is free and public. It tells you which generics are approved and what their TE codes are. But you don’t have to dig through it yourself.
Your pharmacist can tell you which generic you’re getting and whether it’s AB-rated. Ask them: “Is this the same version I was on before?” or “Is this an AB-rated generic?” If they say yes, you’re in good shape. If they say it’s a different manufacturer or you’re switching from a brand to a generic, ask if you should be monitored for any changes.
Many pharmacies now use electronic systems that flag NTI drugs and prevent automatic substitution without a doctor’s note. But not all do. So don’t assume your pharmacist will catch it. Speak up.
Why Do Some Generics Cost So Much Less Than Others?
There can be five or more generic versions of the same drug on the market. One might cost $4, another $12. Why?
It’s simple: competition. The first generic to enter the market often has a slight edge-maybe they have better manufacturing, or their supplier has lower costs. As more companies jump in, prices drop. But sometimes, the cheapest version comes from a manufacturer with less oversight, or one that’s been flagged by the FDA for quality issues in the past.
There’s no official ranking of “best” generic manufacturers. But you can check the FDA’s website for warning letters or inspection reports. If a company has had multiple recalls or violations for poor quality control, it’s worth avoiding-even if their price is tempting.
For most people, choosing a generic from a well-known manufacturer like Teva, Mylan, or Sandoz is a safe bet. These companies have been around for decades and have a track record of compliance.
Switching Generics: When It’s Safe, When It’s Risky
If you’re just starting a medication, it’s usually fine to take the cheapest AB-rated generic. Your body hasn’t adapted to anything yet, so there’s no baseline to disrupt.
But if you’ve been on the same brand or generic for months-or even years-and you’re stable, don’t switch unless you have to. Even a small change in absorption can throw off your rhythm. A 2015 study in Circulation found that patients switching to a new generic version of a heart medication had slightly higher rates of side effects in the first month. Those risks dropped after a few weeks, but why risk it if you’re already doing fine?
Also, if you’ve ever had a bad reaction to a specific generic, even if it was mild-like a headache or nausea-stick with what you know. Your body remembers.
What to Do If You’re Switched Without Warning
Pharmacists are allowed to substitute generics unless your doctor writes “dispense as written.” In most states, they don’t even have to tell you they did it. That means you could be on one generic one month, and a completely different one the next.
If you notice a change in how you feel-more fatigue, dizziness, irregular heartbeat, or mood swings-don’t ignore it. Track it. Write down when it started and what you took. Then call your doctor or pharmacist. Say: “I started feeling different after my last refill. Did they change my generic?”
If you’re on an NTI drug, ask your doctor to write “dispense as written” on your prescription. That forces the pharmacy to give you the exact version you’re on. It’s legal. It’s safe. And it’s your right.
Bottom Line: What to Remember
- Most generics are just as safe and effective as brand-name drugs.
- For most medications, switching between AB-rated generics is fine.
- For drugs with narrow therapeutic indexes (warfarin, levothyroxine, digoxin), consistency matters. Stick with one manufacturer.
- Check the Orange Book’s TE code: AB = safe to switch. B = ask your doctor first.
- If you feel different after a refill, it might be the generic. Don’t brush it off.
- Ask your pharmacist: “Is this the same version I was on?” and “Is it AB-rated?”
- For NTI drugs, ask your doctor to write “dispense as written” on your prescription.
Generic drugs save billions of dollars every year. They’re not a compromise-they’re a smart choice. But smart choices mean being informed. You don’t need to be a scientist to understand the basics. Just ask the right questions, pay attention to how you feel, and don’t let price be the only factor when your health is on the line.
Are generic medications as effective as brand-name drugs?
Yes, for the vast majority of medications, generic drugs are just as effective as brand-name versions. The FDA requires them to contain the same active ingredient, strength, and dosage form, and to be bioequivalent-meaning they deliver the drug into your bloodstream at nearly the same rate and amount. Studies show that differences between brand and generic versions are typically less than 5%. For most people, there’s no noticeable difference in how well the drug works.
Can I safely switch between different generic brands of the same drug?
For most drugs, yes. If the generic has an AB rating in the FDA’s Orange Book, it’s considered interchangeable. But for drugs with a narrow therapeutic index-like warfarin, levothyroxine, or digoxin-switching between generics can cause small changes in blood levels that may affect your health. In those cases, it’s safer to stick with one manufacturer once you’ve found a version that works well for you.
What does an AB rating mean on a generic drug?
An AB rating means the generic drug has been evaluated by the FDA and found to be therapeutically equivalent to the brand-name drug. It has the same active ingredient, strength, dosage form, and bioequivalence profile. AB-rated generics are considered fully interchangeable with the brand and are the safest choice for substitution. Avoid B-rated generics for critical medications unless your doctor specifically recommends it.
Why do some generic drugs cost so much more than others?
Price differences come down to competition and manufacturing. The first generic to enter the market often charges more. As more companies produce the same drug, prices drop. But sometimes, higher-priced generics come from manufacturers with better quality control, stricter testing, or more reliable supply chains. The cheapest option isn’t always the best-especially if the manufacturer has a history of FDA warnings or recalls.
Should I ask my doctor to write “dispense as written” on my prescription?
If you’re taking a drug with a narrow therapeutic index-like warfarin, levothyroxine, or digoxin-yes. This instruction tells the pharmacy not to substitute a different generic without your doctor’s approval. It helps prevent accidental switches that could affect your blood levels. Even if you’re stable on a specific generic, this ensures you stay on the same version. It’s a simple step that can prevent serious side effects.
How do I know if my generic has changed?
Check the name on the bottle. Generic drugs are labeled with the manufacturer’s name (like Teva, Mylan, or Sandoz). If it’s different from your last refill, it’s a different version. Also, look at the shape, color, or imprint on the pill-those often change between manufacturers. If you feel different after a refill-more tired, dizzy, or off-you might have been switched. Call your pharmacist and ask what version you received.
Are there any generic drugs I should avoid entirely?
There’s no official list of “bad” generics. But you should avoid generics from manufacturers that have received FDA warning letters for quality issues, such as contamination, poor manufacturing practices, or falsified test results. You can check the FDA’s website for inspection reports and warning letters. If a generic is significantly cheaper than others, ask your pharmacist why. Sometimes, low cost signals lower quality or unreliable sourcing.


Post A Comment